Analysis
of the Distribution Coefficient of Simazine in Organic Soils vs. Non-organic
Soils
Lindsay
Braun
Abstract
Soil samples of
high organic and low organic content were collected in this study to analyze the
distribution behavior of the herbicide, Simazine in different soil types.
Simazine is the active ingredient in the commercial herbicide known as
Princep Calibur 90. High Performance Liquid Chromatography was used to analyze
the herbicide absorption capacity of the soil samples. Results show that the amount of herbicide absorbed by the
soil varies linearly with the fraction of organic carbon. Soils that are highly organic compared to those with low
organic matter content retain the herbicide to a greater extent.
The determination of the extent of absorption of the herbicide by
different kinds of soil is important since the smaller amount of absorption by
the soil increases the risk of contamination of the groundwater supply.
Introduction
The
issue of contaminants in the groundwater supply has peaked as an environmental
issue, especially of those contributed by organic crop herbicides.
The movement of the contaminant into groundwater is controlled by the
distribution coefficient (Kd) of soils, which is a measure of amount
of interaction between the herbicide and the makeup of separate soil types,
(Dolan, 1998),
Kd
= S (equation
1)
C
where S is the
amount of herbicide retained in the soil and C is the amount of herbicide
absorbed by the water supply. The
amount of herbicide absorbed by the soil depends on the fraction of organic
carbon within the soil (Dolan, 1998). Assuming
that the amount of herbicide retained
by the soil is directly proportional to its content of organic carbon, Kd
can be expressed in terms of the soil�s organic
carbon content:
Kd
= Koc x foc
(equation
2)
where Koc
is the organic carbon-water distribution coefficient and foc is the
fraction of organic carbon in a given type of soil.
The plot of Kd versus foc is linear; Koc
is the slope of the linear plot. Anything
other than a linear plot would imply that the amount of chemical absorbed into
the groundwater is affected by the non-organic makeup of soil (Dolan, 1998).
The herbicide used in this experiment is Simazine
(2-chloro-4,6-bis-ethyl-amino-s-triazine), which is the active ingredient of
Princep Calibur 90. The chemical makeup of Princep is closely related to that of
Atrazine (2-choro-4-ethylamino-6-isopropylamino-6-isopropylamino-s-trazine).
The concentrations of Atrazine found in the groundwater supply has been
under observation in numerous experiments.
Even though Princep is widely used in agricultural settings just as is
Atrazine, it had not been given the attention that Atrazine had as an
environmental hazard. This study
confirms that Princep seems to react similar to Atrazine with the organic matrix
of soils.
In this study, High Performance Liquid Chromatography (HPLC) is used to
evaluate the amount of Simazine absorbed by the organic components of soil.
Three soil types are analyzed. The
two organic soil samples used are the commercial topsoil and the soil gathered
from outside of Vandalia, IL. Sand
is chosen to represent the non-organic soil used in this study.
The procedure followed in this experiment is similar to that of Dolan,
Zhang, and Klarup who used the herbicide, Atrazine (Dolan, 1998). The purpose of
this experiment, in reference to the experiment involving Atrazine, is to see
how closely these two herbicides are related.
This study confirmed that the extent of groundwater contamination by
Princep depends on the local soil type (i.e. the organic content of soil),
similar to the experimental results obtained with the respect to Atrazine.
Materials
Equipment used:
Buck Scientific BLC-20-UV-VIS Integrated HPLC System
Drying Oven
Degassing Apparatus
Whatman Syringe Filters (with 0.2 micrometer size membrane)
Chemicals used:
Mobile Phase: (1:1), Acetonitrile :
Water
Princep (56 ppm in Acetonitrile/Water)
Method
Three soil samples are gathered including two rich in organic (commercial
topsoil and farm ground from Vandalia, IL) and one rich in
non-organic components (sand). Ten
grams of each soil sample is heated overnight at one hundred five degrees
Celsius, then for ten hours at two hundred fifty degrees Celcius, to determine
the percent moisture and the fraction of organic matter, respectively.
To analyze the amount of Simazine retained in the soil, each of the three
soil samples are treated with 50 mL of 56 ppm solution of Princep in
acetonitrile/water mixture. During
this procedure, the solutions are swirled continuously for ten minutes followed
by occasional swirling for twenty-four hours.
Afterwards, the solutions are centrifuged for ten minutes followed by
filtration, first, with #42 Whatman filter paper and, then, using a syringe
filtration technique. Finally, all
of the samples are analyzed by HPLC. Reference
solutions for each soil sample without Princep are also analyzed by HPLC for
comparison.
Data:
Chromatograms for different soil types in the presence and absence of
Princep are shown below:
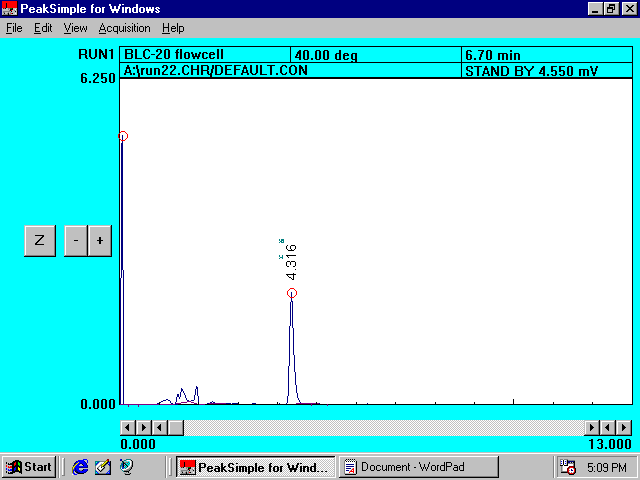
Blank
Solution (without soil) Containing Princep*

Chromatogram
I
*
The only
peak measurable (with a good signal/noise ratio) appeared at the retention time
of 4.316 minutes. The peak was
assigned to Princep.
Filtered
Sand Solution Containing Princep*
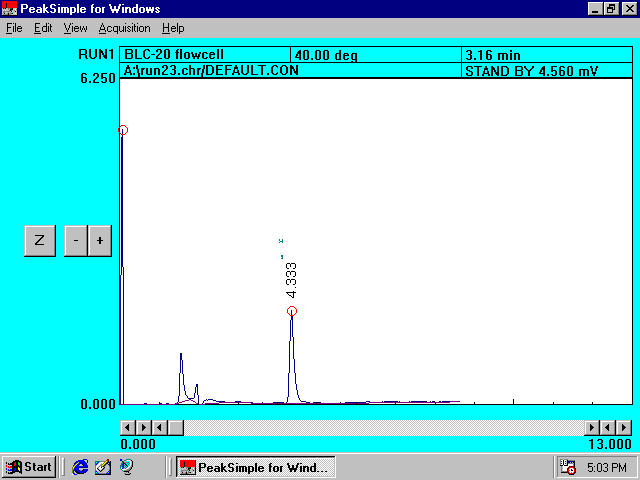
Chromatogram
II
*This
chromatogram analyzes the amount of Princep not retained by sand.
The peak area eluted at 4.333 minutes is directly proportional to the
amount of Princep present in the solution.
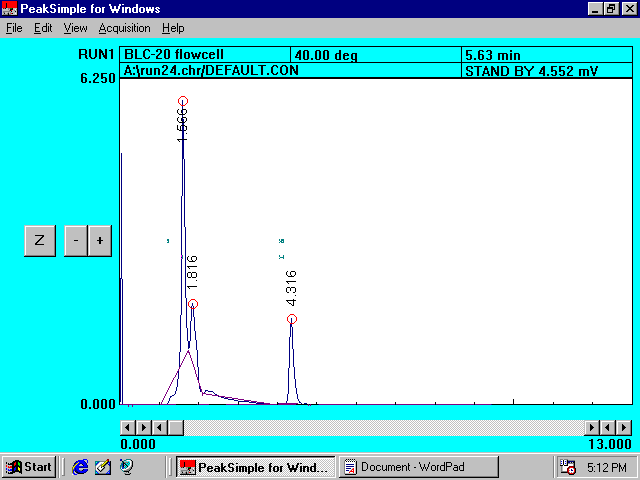
Filtered
Farm Ground Solution Containing Princep*
Chromatogram
III
*Princep
peak area at 4.416 minutes is smaller than that for �sand� which indicates
that more amount of herbicide is retained by �farm ground� than by
�sand�.
Filtered
Topsoil Solution Containing Princep*
Chromatogram
IV
*The Princep peak
area at 4.316 minutes is slightly smaller than that for �farm ground� which
indicates that more amount of herbicide is retained by �topsoil� than by
�farm ground�.
Filtered
Sand Solution without Princep*
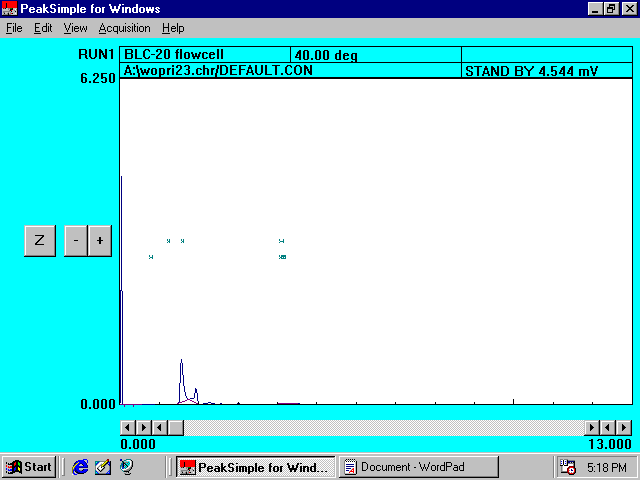
Reference
Chromatogram I
* Princep peak at
4.316 minutes is not observed.
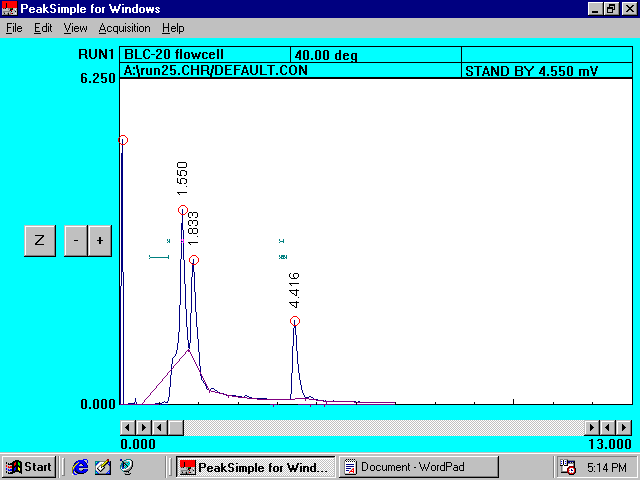
Filtered Farm Ground Solution without Princep*
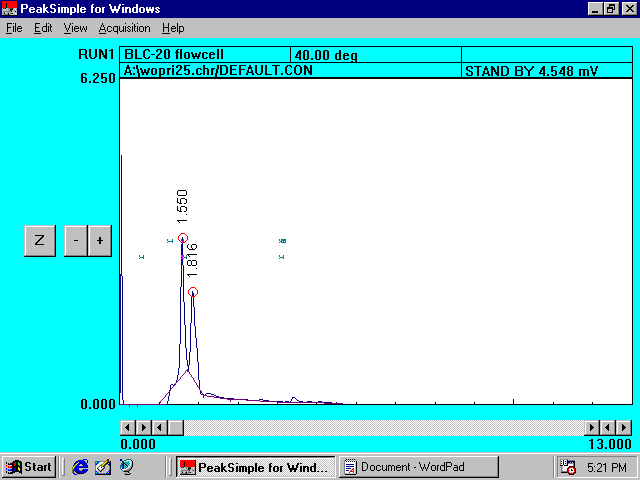
Reference
Chromatogram II
*Peaks at 1.550 and 1.816 minutes represent the organic compounds present in �farm ground� soil.
Filtered
Topsoil Solution without Princep*
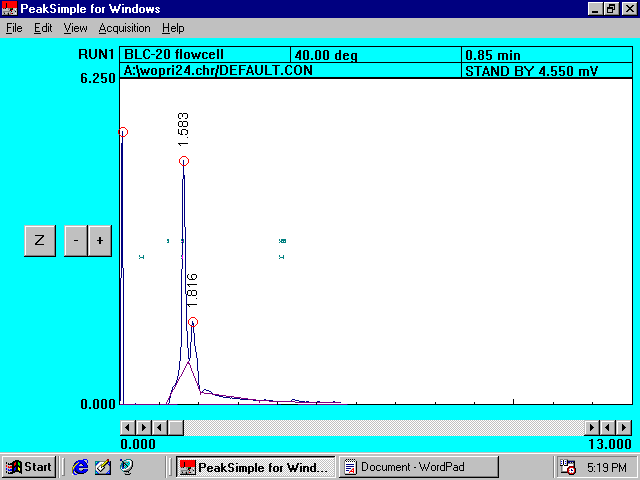
Reference
Chromatogram III
*
It is clear that the amounts of organic acids present (at 1.583 and 1.816
minutes) are much larger than those observed in �farm ground�.
Results
The amount of mass lost upon heating for different soil samples are given
in Table I. Determinations of the percent moisture content and the
fraction of organic matter are based on the mass losses at one hundred five
degrees Celcius and two hundred fifty degrees Celcius, respectively.
The fraction of organic carbon (foc) was calculated by
assuming that about 0.58 of the fraction of organic matter (fom)
consists of organic carbon (Dolan, 1998).
Table
I
|
Soil
Type |
Initial Mass |
Mass
After Heating at 105o C |
Mass
After Heating at 250o C |
%
moisture |
fom
(fraction
of organic matter) |
foc (fraction
of organic carbon) |
|
Sand |
10.05g |
10.02g |
9.98g |
0.299% |
.0039 |
.0023 |
|
Farm
Ground |
10.00g |
7.09g |
6.76g |
29.10% |
.0465 |
.0270 |
|
Topsoil |
10.02g |
7.92g |
7.44g |
20.96% |
.0606 |
.0351 |
As indicated in Table I, the % moisture present in the soil increases in
the order: sand < topsoil < farm ground. Also, the fraction of organic matter (fom) and the
fraction of organic carbon (foc) are found to be highest and lowest
in �topsoil� and �sand,� respectively.
Table
II gives the results of HPLC analysis (peak areas and retention times in
minutes) for different soil samples after treatment with Princep.
Table
II
Retention
Times (min)
|
Samples
containing Princep |
Peak
1a (humic
acids) |
Peak
1b (fulvic
acids) |
Peak
2 (Princep) |
|
Blank |
------- |
------ |
4.316 (area:
15.662) |
|
Sand |
------- |
------ |
4.333 (area:
13.730) |
|
Farm
Ground |
1.550 (area:
26.400) |
1.816 (area:
16.277) |
4.416 (area:
12.843) |
|
Topsoil |
1.666 (area:
21.628) |
1.816 (area:
8.360) |
4.316 (area:
12.669) |
As indicated by the peak areas of Princep in Table II, the amount of
Princep not retained by soil increases in the order:
topsoil < farm ground <sand.
Results of HPLC analysis of
reference soil solutions without Princep are shown in Table III for comparison
of retention times.
Table
III
Retention
Times (min)
|
Samples
without Princep |
Peak
1a (humic
acids) |
Peak
1b (fulvic
acids) |
|
Sand |
------- |
------- |
|
Farm
Ground |
1.550 |
1.816 |
|
Topsoil |
1.583 |
1.816 |
Calculations of the Distribution Coefficient, Kd for different
soil types:
1.
Determining the amount of Princep retained in the soil (S):
First, the amount of Princep in the solvent (C) was calculated using the
chromatograms according to the following equation:
C = peak
area of soil sample x amount of Princep in blank
peak
area of the blank
Then,
S, the amount of Princep retained in each soil sample is calculated using the
equation:
S
= (Ci � C) x 50mL
Msoil
where Ci
is the total amount of Princep added to the soil samples in 50 mL of solvent, C
is the amount of Princep not retained by the soil and Msoil is the
dry soil mass.
Kd is
found by taking the ratio of the amount of Princep retained by soil (S) to
the amount absorbed by water (C).
The
results are shown in Table 4.
Table
IV
|
Soil
Sample |
C (ppm) (amount
of Princep absorbed by water) |
S
(ppm) (amount
of Princep retained by the soil) |
Kd
(S/C) |
foc (fraction
of organic carbon) |
|
Sand |
2.450
x 10-5 |
3.448
x 10-6 |
0.140 |
0.0023 |
|
Farm
Ground |
3.238
x 10-5 |
7.109
x 10-6 |
0.220 |
0.0271 |
|
Topsoil |
2.860
x 10-5 |
6.755
x10-6 |
0.236 |
0.0351 |
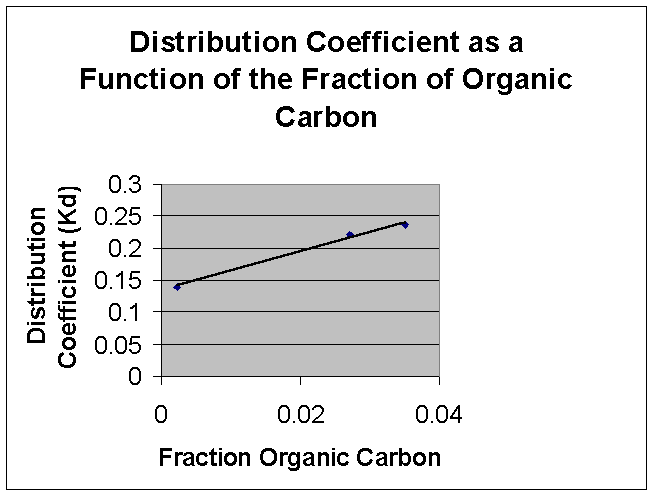
The
linear relationship between Kd and the fraction of organic carbon is
shown on the graph below:
Discussion
The data gathered in this experiment supports the theory that soil with
more organic carbon absorbs organic herbicides better than a soil with little or
no organic carbon. The
topsoil used in this experiment contains the highest concentration of organic
carbon. The farm ground contains just slightly less organic carbon
than the topsoil. The sand contains
hardly any organic carbon. The plot
of the distribution coefficient versus the fraction of organic carbon appears to
be linear, supporting the idea that the organic carbon content of soil increases
the amount of herbicide absorbed into the soil.
Peak 2 on the chromatograms represents Princep in the solution.
The peak area is proportional to the amount of Princep not retained by
the soil sample. As shown in Table
II, Princep peak area decreases as the organic content of soil increases.
Peak 1a represents humic
acids, and peak 1b represents
fulvic acids. These two organic
components are portions of Humus (decomposed plant and animal matter), which are
soluble. Humic acids are present in
a greater amount than fulvic acids in grassland soils (http://www.ar.wroc.pl/~weber/kwasy2.htm).
This fact is supported by the larger peak area of humic acids relative to
that of fulvic acids on the chromatograms of topsoil and farm ground samples.
Also, comparison of the chromatograms (farm ground and topsoil) indicates
that topsoil contains a much larger ratio of humic acids than farm ground does.
This study shows that the groundwater supply could be saved from
agricultural runoff if the soil type absorbs most of the herbicide.
The soils containing high organic carbon content absorb most and,
therefore, are the most likely to prevent contamination of the groundwater
supplies.
Works Cited
Chefez,
B., Chen, Y., Clapp, C., Hatcher, P. �Characterization
of Organic Matter Soils.� Soil Science of America Journal. 2000. (online)
Dolan, E., Zhang,
Y., Klarup, D. �The Distribution
Coefficient of Atrazine with Illinois
Soils.� Journal of Chemical
Education. Vol. 75 No. 12. 1998.
Meloan, Clifton.
�Chemical Separations: Principles,
Techniques, and Experiments.�
Jon Wiley and Sons Inc., 1999.
Pederson, T. L.
�Pesticide Residues in Drinking Water.�
June 1997.
(online)
Pike, David R.
�Reducing Herbicide Movement to Surface and Groundwater.�
December 11, 1998. (online)
Roeth, F. W.,
Comfort, S. D. �Questions and
Answers About Atrazine.� April
1996. (online)
Vitosh, Maurice L.
and Jacobs, Lee W. �Nutrient
Management to Protect Water Quality.� January 1996. (online)
�What are Humic
Acids?� http:
www.horizonag.com/art_2.htm.
1993.
�Properties of
Humic Substances.� http:
ar.wroc.pl/~weber/kwasy2.htm.
©