The Detection of Nonheme Iron Proteins in Polyacrylamide Gels
Sarah B. Cunningham
INTRODUCTION
Hereditary Hemochromatosis (HH) is an inherited disorder of iron metabolism characterized by excess dietary iron absorption and iron deposition in several tissues, particularly the liver and small intestine (1). HH is a prevalent genetic disorder, affecting up to one in 250 individuals of European descent (2). Clinical consequences include hepatic failure, hepatocellular carcinoma, diabetes, cardiac failure, impotence and arthritis (1). Liver disease resulting from iron toxicity is the major cause of death in hemochromatosis (3).
Insight into the molecular basis of HH first came almost 25 years ago when the phenotype was shown to be linked to the human leukocyte antigen (HLA) complex (2). This observation led to the identification of the causative gene, HFE, in 1996 by positional cloning (4). The HFE gene encodes for a protein that helps regulate iron uptake by enterocytes, intestinal cells, but the mechanism by which this occurs is not fully understood. Several mutant forms of the gene have been identified and are thought to be associated with the development of hemochromatosis (5). In populations of Northern European descent, 80% of hereditary hemochromatosis is related to the Cys282Tyr mutation in the HFE gene. In both humans and mice, this mutation causes iron-loading phenotypes suggesting that the function of HFE gene product is required for iron homeostasis (6).
Full expression of hemochromatosis only occurs in homozygotes (7). In contrast, heterozygotes may demonstrate minor biochemical abnormalities of iron status, but do not develop a progressive increase in body iron stores of the order seen in homozygotes (8). Even in homozygotes, the phenotypic expression of the disease varies considerably due to environmental and dietary factors (7).
Under normal physiologic conditions, the absorption of iron from dietary sources is highly influenced by the body�s requirement for the mineral (5). If body iron stores are depleted, dietary iron is more readily absorbed from the gastrointestinal tract. If, on the other hand, iron stores are plentiful, enterocytes (intestinal cells) absorb iron less efficiently, thereby helping to avoid iron overload and its toxic effects. In HH, however, iron continues to be absorbed by the enterocytes at a high rate despite the presence of abundant iron stores in the liver and other tissues (5). In hemochromatosis the regulation of iron absorption from the small intestine is defective and dietary iron absorption is high and inappropriate for the levels of body iron stores (9). The pathogenesis of HH is thought to involve defects in the mechanism controlling duodenal iron absorption, but the nature and the primary site of the defect is still unrecognized.
Transferrin, transferrin receptor and ferritin are the major proteins involved in the control of cellular iron metabolism. In particular, ferritin, the major iron-storage protein, has been viewed as the protein that physiologically plays a key role in the control of duodenal iron absorption (9). Ferritin has been proposed as a possible �acceptor� which would store the iron absorbed in excess (10). Therefore, it is thought that ferritin may be involved in the pathogenesis of HH (9).
Transferrin, ferritin, and ferredoxin are all considered nonheme iron proteins (9). Nonheme iron proteins are a family of proteins, each with an iron-sulfur (Fe-S) center that consists of iron and sulfur atoms complexed with cysteine groups of the protein (11). The iron atoms of the Fe-S centers are the actual electron acceptors and donors of the iron-sulfur proteins. Each iron atom alternates between the Fe+3 (ferric) and Fe+2 (ferrous) states as the centers are oxidized and reduced. Heme iron proteins are like the nonheme iron proteins, also containing iron, but are a part of a porphyrin prosthetic which is recognized as a component of hemoglobin (11).
Ferritin is an important iron storing protein and its synthesis is selectively stimulated in the presence of iron (10). Ferritin is a well characterized protein consisting of 24 polypeptide subunits forming a shell around a central cavity in which up to 4500 iron atoms may be stored (12). Most ferritin shells exist as 2 subunits, heavy (H) and light (L) chains (10). Ferritin is found in most tissues. However, the composition of ferritin varies in a consistent and tissue-specific way. For example, liver contains ferritin that is predominantly of the L subunit type, whereas heart and small intestine contains ferritin rich in the H subunit. According to Rucker et.al (13), it is thought that the H and L ferritin subunits may play different and complementary roles within the protein. The H subunit has ferroxidase activity which catalyzes the oxidation of Fe2+ (ferrous) to Fe3+ (ferric), and enhances the rate of iron uptake into the ferritin molecule. The L subunit increases the rate of iron core formation and is involved in protein stability (10).
Mouse cells and mouse models have been widely used to study iron homeostasis in health and disease. For the most part, however, biological inferences concerning ferritin function and iron metabolism in these models have been based on analogy to human ferritin (13). Indeed, substantial sequence similarity exists between mouse and human ferritin subunits; mouse L and human L exhibit 82% similarity, whereas human and mouse ferritin H are 93% identical (13).
The study of ferritin is very important in relation to hereditary hemochromatosis. Ferritin is thought to play a key role in the control of duodenal iron absorption (9). In HH this control of duodenal iron absorption is defective and causes inappropriately high levels of iron overload. Mice with targeted mutations in the HFE gene can be used as an important tool for understanding genetic disorders such as hemochromatosis. The ferritin protein is found in most iron complexes from liver and small intestine samples from HFE linked hemochromatosis (1). However, the detection of other nonheme proteins other than H and L ferritin that are found in the iron complex of HFE mice are of great interest. The purpose of this experiment was to detect and identify nonheme proteins found in the iron complex from HFE mice, particularly in the liver and small intestine, and their relation to the H and L ferritin. Other tissues such as the kidney, heart, and spleen were also analyzed for the presence of nonheme proteins. The detection of nonheme iron proteins was achieved in two ways. First was the detection of the presence of iron in protein bands separated by polyacrylamide gel electrophoresis (14). After polyacrylamide gel electrophoresis, the gel was either immunoblotted or stained with Modified Perls. MALDI MS (matrix assisted laser desorption ionization time-of-flight mass spectrometry) analysis was used to determine the identity of the unknown nonheme proteins by the protein�s molecular weight distributions and the structure of the protein (15). The heterogeneity of ferritin was also analyzed. In recent papers, it has been suggested that the H and L subunit are heteropolymers (13). The work that was performed in this experiment supported the H and L subunits are homopolymers rather than heteropolymers.
METHODS AND MATERIALS
All materials and methods described below took place at the Cardinal Glennon Pediatric Research Institute located in St.Louis, Missouri. The laboratory is strictly for iron metabolism research and is affiliated with St.Louis University School of Medicine. All laboratory work was performed under the direction and assistance of Dr. Robert Fleming.
Animal Models
Mice were housed in the barrier facility at Cardinal Glennon Pediatric Research Center and maintained on a 4% carbonyl diet. All mouse production and experimentation were in compliance with the guidelines of the Institutional Animal Care and Use Committee. A total of fifty mice of known genotype were used in this study. DTA mice are iron deficient and were used as controls for the experiment. The TFR2 mice have the transferrin receptor 2 protein that is responsible for iron loading. The wild-type mice are have normal levels of iron and do not have the transferrin receptor 2 present. Because iron accumulation is progressive, the size of the iron stores is dependent upon age. For this reason the experimental and control animals of the same age were used for comparison (9). At 4 weeks of age, the animals were fasted for 14 h before being sacrificed. Arterial blood, 1-2 cm of small intestine (duodenum), heart, spleen, and kidney were obtained. Tissue samples were immediately frozen in liquid nitrogen. Samples were labeled and stored in a -90EC freezer. Some of the samples were obtained for histology.
Histochemistry
Samples obtained from the heart, duodenum, spleen and liver were sectioned at section thickness on fixed embedded paraffinized histological slides. The tissues were iron stained by following the modified Perl�s technique and were counterstained with nuclear fast red (16).
Homogenization and Sonication Procedure
Liver, small intestine, heart, spleen, and kidney samples were homogenized in cold phosphate buffered saline (PBS) with a protease inhibitor, 1 ml of PBS per 1 g of tissue. Samples were homogenized for 20 s each. After homogenization, samples were sonicated for four times for 15 s each and placed immediately on dry ice. Sonication is a useful method that subjects the cells to short, intense treatments with ultrasound. As a result, it breaks the cell walls into sizes that will not affect the viscosity of the samples (17).
Enrichment of Protein Samples and Bradford Protein Assay
The tissue samples were enriched based on the procedure of Fleming et al. (3). The samples were spun at 20,000 rpm for 30 min. After this spin, the supernatant was collected and the tubes were washed and the pellet was discarded. The second speed was 50,000 rpm for 30 min. The pellet was collected and suspended in extraction buffer. The extraction buffer was comprised of 250 mM Tris (hydroxymethyl-aminomethane) and 250 mM EDTA (ethylenediaminetetraacetic acid). Most pellets were very small and were thus extracted in about 40-50 :l of extraction buffer.
Next Bradford Protein assay was performed to test for the amount of protein present in the samples. This is not an assay to tell what kind of protein is present but simply how much is present. According to the Bio Rad Protein Assay protocol, a 1:4 dilution of the Bio Rad dye reagent was performed first (18). 1 ml of this dilution was added to each cuvette. Protein standards were prepared in the same buffer as the samples were assayed. A standard curve was made using bovine serum albumin (BSA) with concentrations of 0, 250, 500, 1000, 1500, and 2000 :g/ml. Small intestine, liver, kidney, spleen and heart samples were added to the dilution of Bio Rad reagent. Samples were mixed well with the vortex machine before read in the spectrophotometer. The spectrophotometer was blanked and samples were read at 500 nm. Calculations were performed to figure the standard curve and the amount of protein present in the small intestine and liver.
Polyacrylamide Gel Electrophoresis
Sodium dodecylsulphate polyacrylamide gels electrophoresis (SDS- PAGE) was conducted using an apparatus from Bio-Rad Laboratories (vertical slabs). Proteins are separated on the basis of their molecular mass using SDS-PAGE. Either 5% or 10% SDS -PAGE was prepared according to the type of experiment being performed. Often 5% gels were ran when staining with modified Perls stain or extracting proteins from the gel. The 10% gels were ran when performing a western blot and reacting the blots with antibodies. Following the protocol of Harlow and Lane (17), the composition of the running gel was comprised of distilled water, 1.5 M Tris HCL pH 8.8, 30% acrylamide/0.8% bis acrylamide, 10% SDS, and 10% APS (ammonium persulfate). The stacking gel was comprised of distilled water, 0.5 M Tris HCl pH 6.8, 30% acrylamide/0.8% bis acrylamide, 10% SDS and 10 % APS (17). Samples were electrophoresed at 110 mV for approximately 45 min.
Potassium Ferricyanide Iron Staining (Modified Perl�s Stain)
The Modified Perl�s stain is commonly used in histochemistry to detect the presence of iron in tissues (19). A very distinct dark brown stain will represent the presence of iron. Similarly, the same protocol that was followed for histochemistry was adjusted and used to iron stain the bands found in SDS PAGE. Stock solutions A (1 ml HCL in 24 ml distilled water) and B (1g of potassium ferricyanide in 25 ml of water) were mixed together and applied directly to the gels. The gels were stained for 15 min and then rinsed with distilled water. Next, the DAB (diaminobenzide) substrate was prepared and applied to the gels. Iron bands will appear dark brown after approximately 30 min with DAB substrate.
Immunoblotting (Western Blotting)
Immunoblotting was another alternative for the detection of the H and L chain ferritin. Immunoblotting involves polyacrylamide gel electrophoresis of a protein specimen followed by transfer of the separated protein to nitrocellulose sheet, also called polyvinyldifluoride (PVDF) membranes. Spots or bands can be detected primarily by immunoreacting or by staining the membrane. This is a slightly more complicated procedure but allows detection and identification of specific proteins. Following the protocol by Hames and Rickwood (20), one PVDF membrane (6 x 9 cm) was soaked in methanol for 2 min then washed twice with distilled water and soaked in lower running buffer. Six Whatman filter papers (6 x 9 cm) were also soaked in lower running buffer. With completion of electrophoresis, three Whatman filter papers, one PVDF membrane, the polyacrylamide gel, then three more Whatman papers were stacked in the Bio Rad blotting apparatus. The blotter ran at 110 mA for 50 min for two membranes and 55 mA with one membrane for 50 min. After the blotter finished blotting, the PVDF membrane was removed and set in lower running buffer until staining or immunoreacting was performed. The polyacrylamide gel can also be removed and stained with Coomassie Brilliant blue to detect the presence of protein bands.
Immunoreacting and Chemiluminescence
Protein bands from the western blot are often visualized by treating the nitrocellulose sheet with a solution of antibodies (11). Because of the specificity of the antibody-antigen reaction, the antibody will bind only to places on the nitrocellulose paper where the protein of interest is located (20). In this way, the protein can be localized to a specific band in the polyacrlyamide gel, and the amount of protein in that band can be quantified. The immunoreacting and chemiluminescence procedures were followed as described by Hames and Rickwood (20). After immunoblotting, the PVDF membrane was washed with Casein blocking solution for 15 min. The membrane was cut in half and one half was reacted with a primary antibody anti-ferritin L-chain and the other half with anti-ferritin H-chain. The primary antibodies were diluted 1:3000. The membrane was incubated overnight at 4EC. The incubated membrane was washed five times at 10 min intervals with tris-buffered saline tween-20 (TBST). After washing, a secondary antibody, Goat anti-Rabbit IgG, was diluted 1:6000 and was applied and incubated for 1 h. After reacting with secondary antibody, the membrane was washed five times at 10 min intervals with TBST. The next step and very crucial part of the procedure was the detection of protein bands with chemiluminescence. Luminol was diluted 1:1000 and applied directly to the membrane for 90 s and then removed immediately. The membrane was placed against an X-ray film, Kodak direct exposure film.
MALDI MS (Matrix assisted laser desorption ionization time -of -flight mass spectrometry)
One nonheme iron-containing protein was found during the above procedures (see Results), and MALDI MS was performed to determine the identity of the unknown nonheme protein. MALDI-MS is a powerful tool for determining molecular-weight distributions and structures of synthetic organic polymers (15). The bands in the polyacrylamide gel that contained the unknown protein were excised for the analysis by the MALDI-MS. Access to this to type of equipment was unavailable. The samples were sent to Columbia University to be analyzed.
RESULTS
Histochemistry reveals dark iron staining in the liver and duodenum
|
|
 Modified Perl�s was applied to fixed paraffin embedded histological sections
from the duodenum and liver tissues of the TFR2, DTA, and wild-type mice. A dark
brown stain illustrated the presence of iron when using the Modified Perl�s
stain with the reaction of the DAB (diaminobenzide) substrate. The DTA mice
duodenum and liver demonstrated having no iron staining present. Whereas, the
TFR2 mice had a large amount of iron present particularly in the liver, and the
wild-type mice had a smaller amount of iron staining present. The liver and
duodenum stained with the modified Perl�s and DAB substrate (Fig. 1-3)
illustrated the great abundance of iron that was present in the TFR2 mice. Other
tissues that were stained included the spleen, heart, and kidney that also
revealed to have iron present.
Modified Perl�s was applied to fixed paraffin embedded histological sections
from the duodenum and liver tissues of the TFR2, DTA, and wild-type mice. A dark
brown stain illustrated the presence of iron when using the Modified Perl�s
stain with the reaction of the DAB (diaminobenzide) substrate. The DTA mice
duodenum and liver demonstrated having no iron staining present. Whereas, the
TFR2 mice had a large amount of iron present particularly in the liver, and the
wild-type mice had a smaller amount of iron staining present. The liver and
duodenum stained with the modified Perl�s and DAB substrate (Fig. 1-3)
illustrated the great abundance of iron that was present in the TFR2 mice. Other
tissues that were stained included the spleen, heart, and kidney that also
revealed to have iron present.
|
|
|
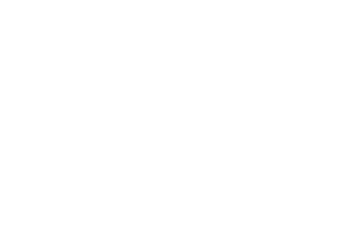
Figure 3. Modified Perl�s stain of the TFR2 liver with DAB substrate. Demonstrates the large abundance of iron present in the liver and particularly around the central veins. Photomicrographs were taken through an Olympus BX50 microscope. Magnification 200X. |
|
|
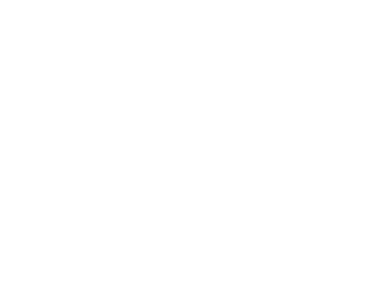
|
Figure 2. Modified Perl�s stain of the TFR2 liver without the DAB substrate reaction and counter stained with nuclear fast red (kernechtrot). Photomicrographs were taken through an Olympus BX50 microscope. Magnification 200X. |
Histochemistry led to experimentation with polyacrylamide gel electrophoresis
As described in Materials and Methods, polyacrylamide gels were stained with modified Perl�s and DAB substrate to determine if iron proteins could be detected using this method. Various experiments were run using the Perl�s stain on polyacrylamide gels using different tissue samples including the liver, duodenum, kidney, spleen, and heart. However, most of the experiments primarily focused on the liver and duodenum. In the presence of iron, distinct dark brown bands were present. A low range molecular weight marker was used to determine the molecular weight of the bands. According to Boyd et al., the molecular weight of the ferritin H subunit is 21 kDa and the L subunit is19 kDa (22). In Figure 4, liver and duodenum samples from iron deficient, TFR2 and wild-type mice were used. The lane with the molecular weight marker had been cut off and stained with coomassie brilliant blue and is not present in figure 4. The DTA mice, used as a control illustrated to have no bands present for the detection of iron proteins. Whereas, the TFR2 and wild-type liver bands had a molecular weight of about 21 kDa, and the wild-type duodenum with a molecular weight of 19 kDa which helped conclude that the liver was primarily L ferritin in TFR2 and wild-type mice, and the duodenum was primarily H ferritin in the wild-type mouse. An interesting finding was that the duodenum of the TFR2 had two other bands present The middle band on the TfR2 duodenum had a molecular weight of 21 kDa, L ferritin. The lower band was the 19 kDa band (H ferritin), the middle band was 21 kDa (L ferritin), and the upper band was about 25 kDa. This upper band did not correlate with the L ferritin band and was determined to be an unknown protein. The same experiment was ran several more times changing different variables and the findings and results were the same.
Characterization of the duodenum 55 kd band by MALDI MS
After performing many experiments and being unable to characterize the duodenum 25 kDa band, further experimentation was performed to determine the unknown protein. As according to the Materials and Methods, the bands of the unknown protein were excised for analysis by Kendrick Laboratories at Columbia University to be characterized by the MALDI MS.
It was concluded from Kendrick Laboratories that the unknown protein of the duodenum matched human keratin 10, Swiss Protein # P13645, 26 kDa. This keratin is common especially in the 25-35 kDa range. There are other peaks from the mass spectrometer reading that matched mouse keratin 10 , Swiss Protein # P02535. However, the match for the human keratin 10 was much better.
Immunoblotting (Western blotting) and the detection of H and L ferritin
|
|
DISSCUSSION
Applying the modified Perl�s stain to embedded paraffin histological sections illustrated the great abundance of iron that was present particularly in the TFR2 mice duodenum and liver. In the duodenum the majority of the iron staining was found at the lining of the epithelium columnar cells of the villi (Figure 1). The liver contained a large quantity of iron represented by the hepatic lobules staining entirely brown (Figure 1). There is not a histological stain that can differentiate the proteins, particularly H and L ferritin, found in the iron complex of the duodenum and liver. Therefore, polyacrylamide gel electrophoresis was performed to primarily detect proteins found in the iron complex by applying the modified Perl�s stain with DAB.
The ability of iron to catalyze the oxidation of diaminobenzoic acid provides the basis for detecting iron-containing protein bands on polyacrylamide gel (14). The results from the polyacrylamide gels that were stained with modified Perl�s and DAB substrate clearly detected the presence of proteins in the iron complex. As shown in Figure 4, this correlates with Boyd et.al. findings showing the liver has a greater molecular weight than the small intestines lower band. The molecular weight of the bands found in the TFR2 and wild-type liver, 21 kD, matched the molecular weight of L ferritin. It is important to note the heavier and darker stained band found in the TFR2 liver. This helps illustrate that the TFR2 has a greater amount of iron present than the wild-type. The molecular weight of the lower band in the TFR2 and wild-type duodenum, 19 kD, matched the molecular weight of the H ferritin. The TFR2 duodenum also had L ferritin present found in the middle band and the unknown protein from the upper band that was sent to Kendrick Laboratories was characterized to be keratin. This result seems to suggest that there was possible contamination from either the skin from a human, or hair or skin from the mouse that contaminated the tissue sample. The contamination was most likely to have occurred when sacrificing the mice and removing the tissue.
The results from immunoblotting helped conclude that the liver is comprised mostly of L-ferritin, the duodenum of H-ferritin, and the heart was comprised of both H and L ferritin. From this experiment it can be concluded that the liver is a homopolymer of L ferritin, duodenum is homopolymer of H ferritin and the heart is a heteropolymer of H and L ferritin. However, from the SDS PAGE stained with Modified Perl�s and DAB (Figure 4), the results were not consistent with the immunoblotting findings. In Figure 4, liver appears to be a homopolymer of L ferritin in the TFR2 and wild-type mice, but the duodenum of the TFR2 mice appeared to be a heteropolymer containing both H and L ferritin subunits of the iron complex. Previous literature suggests that the liver, duodenum, and heart are all heteropolymers consisting of both H and L ferritin (14). However, the type of experiment and procedures performed to make these conclusions differed. Leong, a scientist performing research on ferritin, made this conclusion by isoelectric focusing (14).
Iron proteins, H and L ferritin, can be detected in polyacrylamide gels stained with modified Perl�s and DAB substrate and also by immunoblotting. The finding that the molecular weight of the ferritin H subunit is 21 kDa and the L subunit is19 kDa was in agreement with Boyd (22). However, further experimentation such as isoelectric focusing needs to be performed to successfully confirm that the duodenum and liver are homopolymers rather than heteropolymers of H and L ferritin.
Acknowledgments
I would like to thank Robert Fleming, M.D. for allowing me to work in his laboratory on this research project and for assisting in stimulating discussions and new ideas on the research project. I would also like to thank other researchers in the laboratory including: Bakht Roshan, M.D., John Ahman M.D., and Mary Migas. Also, thanks to Dr. Ted Anderson for assisting me in preparation of this paper.
LITERATURE CITED
1. Fleming RE, Sly WS. 2002. Mechanisms of iron accumulation in hereditary hemochromatosis. Ann. Rev. Physiol. 64:663-80
2. Levy LE, Montross LK, Andrews NC. 2000. Genes that modify the hemochromatosis phenotype in mice. J. Clin. Invest. 105: 1209-16
3. Fleming RE, Migas MC, Holden CC, Waheed A, Britton RS, et al. 2000. Transferrin receptor2: continued expression in mouse liver in the face of iron overload and in hereditary hemochromatosis. Proc. Natl. Acad. Sci. 97:2214-19
4. Whittaker P, Skikine BS, Covell AM, Flowers C, Cooke A, et al.1989. Duodenal iron proteins in idiopathic hemochromatosis. J. Clin. Invest. 83:261-67
5. Neff LM, Mayer J. 2003. Current directions in hemochromatosis research: Towards an understanding of the role of iron overload and the HFE gene mutations in the development of clinical disease. Nutr. Rev. 61:38-42
6. Simpson RJ, Debnam ES, Laftah NS, Beaumont N, Bahram S, et al. 2003. Duodenal nonheme iron content correlates with iron stores in mice, but the relationship is altered by HFE gene knock-out. Blood. 101:3316-18
7. Hershko C, Konijm AM, Aisen P, eds. 1994. Advances in Experimental Medicine and Biology. Vol.356: Progress in Iron Research, pp. 277-42.New York: Plenum Press
8. Brock JH, Halliday JW, Pippard MJ, Powell LW, eds.1994. Iron metabolism in health and disease, pp. 235-38. London: W.B. Saunders Company Ltd
9. Pietrangelo A, Casalgrandi G, Quaglino D, Gualdi R, Conte D, et al.1995. Duodenal ferritin synthesis in genetic hemochromatosis. Gastroenterology. 108:208-17
10. Gerard B, Farman N, Raja KB, Eugene E, Grandchamp B, Beaumont, C. 1996. Expression of H and L ferritin mRNAs in mouse small intestine. Exp. Cell Res. 228: 8-13
11. Becker WM, Kleinsmith LJ, Hardin J. 2003. The World of the Cell, pp.418-21. San Francisco:Benjamin Cummings
12. Andrews SC, Trefffry A, Harrison PM. 1987. Siderosomal ferritin - the missing link between ferritin and haemosiderin. Biochem. J. 245:439-46
13. Rucker P, Torti FM, Torti SV. Role of H and L subunits in mouse ferritin. 1996. J. Biol. Chem. 271: 33352-57
14. Leong LM, Tan BH, Ho KK. 1992. A specific stain for the detection of nonheme iron proteins in polyacrylamide gels. Anal. Biochem. 207:317-20
15. Wu KJ, Odom RW. 1998. Characterizing Synthetic Polymers by MALDI MS. Anal. Chem. Rep. 1-9. American Chemical Society
16. Kiernan JA. 1999. Histological and histochemical methods, pp. 452-500. Boston: Butterworht Heinemann.
17. Harlow E, Lane D. 1999. Using Antibodies: A Laboratory Manual, pp. 300-495. New York: Cold Spring Harbor Laboratory Press
18. Bio-Rad Protein Assay. 1990. Bio-Rad catalog, pp95-97
19. Chung CC. 1985. A specific iron stain for iron-binding proteins in polyacrylamide gels: application to transferrin and lactoferrin. Anal. Biochem. 148: 498-502
20. Hames BD, Rickwood D, eds. 1990. Gel Electrophoresis of Proteins, pp.395. New York: Oxford University Press
21. Cullen LM, Anderson GJ, Ramm GA, Jazwinska EC, Powell LW. 1999. Genetics of hemochromatosis. Annu. Rev. Med. 50:87-98
22. Boyd D, Vecoli C, Belcher DM, Jain SK, Drysdale JW. 1985. Structural and functional relationships of human ferritin H and L chains deduced from cDNA clones. J.Biol. Chem. 260:11755-61
©