Determination of Cyanide Content in a Variety of Beers by the Microdiffusion Spectrophotometric Method and Analysis of the Correlation between Cyanide Content and Carbohydrate Content
M. Valerie Slaven
The Microdiffusion Spectrophotometric method was used to analyze fourteen commercially available beers for cyanide content. This method includes the microdiffusion of the sample using a Conway cell to free the cyanide from the compound it is bound to as well as separate it from the matrix it is present in. This is followed by a reaction, which utilizes cyanoline blue, a pyridin-pyrazolone reagent, to quantify the amount of cyanide present by analyzing the blue color produced by the reaction in a visible spectrophotometer. The quantification of the amount of cyanide is achieved by first creating a calibration curve using a set of standards between 1.00 x 10-6 M and 1 x 10-5 M. After the amount of cyanide is quantified, the carbohydrate content of the beer and the experimentally determined cyanide content were analyzed for correlation. It was found that there is a direct correlation between the amounts of cyanide and the carbohydrates present in the beer.
Cyanide is present in beer in the form of cyanogenic glucosides, which are produced from the fermentation of the barley in the brewing process. Cyanogenic glucosides are glucosides with constituents that contain cyanide. A Glucoside is a glycoside, a compound derived from monosaccharides, in which the sugar component is glucose. Given that cyanide is present in the beer as cyanogenic glucoside, which is a type of carbohydrate, it seems plausible that the amount of carbohydrates in the beer would be correlated to the cyanide content in the beer.
There are multiple methods available for use to quantify the amount of cyanide present in simple matrices such as water, but there have been few studies published on methods of cyanide detection in other more complex matrices such as beer. There are also a very few studies published on the quantification of cyanide in the cyanogenic glucoside form or the separation of cyanide from cyanogenic glucosides so that it may be quantified as free cyanide. For a method to be effective for cyanide analysis of beer, it must be able to separate the cyanide from both the carbohydrate and other components of the matrix. In this study, the microdiffussion spectrophotometric method as used by Kouichiro et al. is utilized as a quantifying technique for cyanide in beer. [1] The Conway cell is a manual method of microdiffusion that uses a cylindrical microdiffusion cell with two annular compartments. The Conway cell works by adding H2SO4 to the sample in the outer well of the cell. The cyanide is then converted from the cyanogenic glucoside to free cyanide, which then diffuses from the sample�s matrix through the enclosed vapor phase, to a NaOH trap, a much simpler matrix to work with, in the center well of the cell. [5]
After the cyanide is trapped in the NaOH, the solution is buffered to an ideal pH for the indicator, and then the cyanide is chlorinated using chloramines T, N-Chloro-p-toluenesulfonamide sodium salt, to produce cyanogen chloride. After the cyanide is chlorinated, it is treated with cyanoline blue reagent, a mixture of monopyrazolone and bispyrazolone in pyridine and water. The Pyridine separates the cyanide from the chlorine by binding to it while water extracts the cyanide and nitrogen from the pyridine. The compound resulting after cyanide and nitrogen are removed from the substituted pyridine, OHC-CH=CH-CH2-CHO, binds with monopyrazolone and bispyrazolone to produce a blue color. The Spectrophotometric method was then used to create a calibration curve of standard cyanide solutions treated under the same conditions to quantify the amount of cyanide in each sample. This method is the method reported by Kouichiro, et al. and is very similar to the method reported by Zheng, et al..
This method has very low detection limits, making it ideal for the analysis of cyanide in commercially available products. Due to the toxicity of cyanide, there are governmental regulations of commercially available products limiting the amount of cyanide present to very low values.
Cyanoline Blue was obtained from Dojindo and Chloramine-T was obtained from Sigma Aldrich. The Conway cells used were obtained from Fisher Scientific. All other materials used were analytical grade.
Standard solutions of KCN were prepared at 10-6 M to 10-5M concentrations. After the standards were prepared, the standards and the beer samples were treated in the same manner. 1.5 mL of the samples/standards were added to the outer rings of the Conway cells. Then 3.0mL of 1.0M NaOH were added to the center wells of the Conway cells, and the cells were closed with parafilm only leaving a small opening so that 0.75 mL of 10% (w/v) H2SO4 could be added to the outer rings and thus mixed with the samples/standards. After the addition of the H2SO4, the Conway cells were sealed the rest of the way with parafilm and placed in the air incubator for 30 minutes at 40� C. After 30 minutes, the samples were removed from the incubator, and 600 mL of the samples/standards were pipetted into small sample holders. The samples in the sample holders were then treated with 1450 mL pH 6.8 potassium dihydrogen phosphate-potassium monohydrogen phosphate buffer, and 150 �L of 6.25 mg/mL chloramine T solution. These solutions were then set aside to rest for 2 min after which 1.80 mL of 0.27% (w/v) Cyanoline blue in pyridine/water (1:5, v/v) was added to the mixtures. The mixtures were then incubated for 10 min at 40 �C in an air incubator. After the incubation the treated samples were transferred to cuvettes and measured on a Spec-22 Spectrophotometer.
The fourteen beer samples used were separated into four categories of different alcohol content. In each category the percent of alcohol present in the samples was kept constant while the carbohydrate content was allowed to vary. This allowed the variable contributing to the cyanide content to be isolated.
Chart 1: Calibration Curve Concentrations and Average Absorbance
|
Concentration (mM) |
Average Absorbance* |
|
10.00 |
0.026666667 |
|
9.00 |
0.024666667 |
|
8.00 |
0.022333333 |
|
7.00 |
0.019666667 |
|
6.00 |
0.016666667 |
|
5.00 |
0.013666667 |
|
4.00 |
0.011333333 |
|
3.00 |
0.008666667 |
|
2.00 |
0.006333333 |
|
1.00 |
0.002666667 |
*Average Absorbance is the average of three absorbance readings
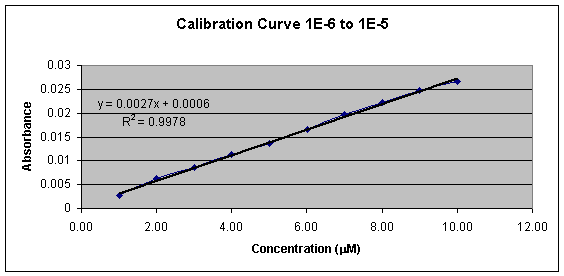
Graph 1: Calibration Curve

Chart 2: 0.40% alcohol content group
|
% alcohol |
Beer |
Carbohydrates (g) |
Average |
Concentration |
Concentration |
|
0.40% |
O'Doul's Amber |
18 |
0.021 |
7.451 |
0.194 |
|
0.40% |
Coors NA |
14.2 |
0.016 |
5.579 |
0.145 |
|
0.40% |
O'Doul's |
13.3 |
0.014 |
5.017 |
0.131 |
|
0.40% |
Busch NA |
12.9 |
0.013 |
4.643 |
0.121 |
*Average Absorbance is the average of two absorbance readings
Chart 3: 4.20% alcohol content group
|
% alcohol |
Beer |
Carbohydrates (g) |
Average |
Concentration |
Concentration |
|
4.20% |
Bud Light |
6.6 |
0.005 |
1.648 |
0.043 |
|
4.20% |
Keystone Light |
5.1 |
0.004 |
1.273 |
0.033 |
|
4.20% |
Miller Light |
3.2 |
0.002 |
0.524 |
0.014 |
*Average Absorbance is the average of two absorbance readings
Chart 4: 5.00% alcohol content group
|
% alcohol |
Beer |
Carbohydrates (g) |
Average |
Concentration |
Concentration |
|
5.00% |
Miller Genuine Draft |
13.1 |
0.014 |
4.830 |
0.126 |
|
5.00% |
Pabst Blue Ribbon |
12.01 |
0.011 |
3.894 |
0.101 |
|
5.00% |
Budweiser |
10.6 |
0.009 |
2.958 |
0.077 |
|
5.00% |
Ice House |
8.7 |
0.007 |
2.396 |
0.062 |
*Average Absorbance is the average of two absorbance readings
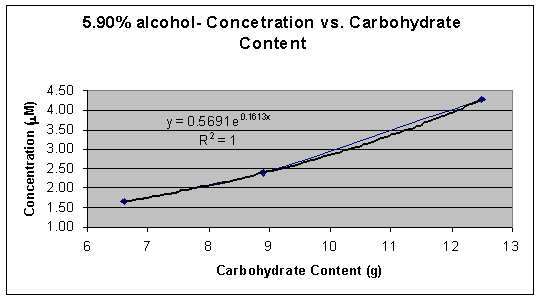
Chart 5: 5.90% alcohol content group
|
% alcohol |
Beer |
Carbohydrates (g) |
Average |
Concentration |
Concentration |
|
5.90% |
Busch Ice |
12.5 |
0.012 |
4.269 |
0.111 |
|
5.90% |
Natural Ice |
8.9 |
0.007 |
2.396 |
0.062 |
|
5.90% |
Keystone Ice |
6.6 |
0.005 |
1.648 |
0.043 |
*Average Absorbance is the average of two absorbance readings
Graph 2: 0.40% alcohol � Concentration (mM) vs. Carbohydrate Content (g)
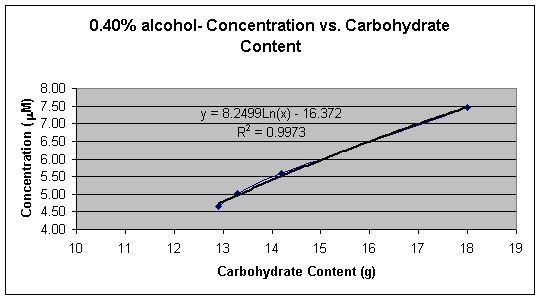
Graph 3: 4.20% alcohol � Concentration (mM) vs. Carbohydrate Content (g)
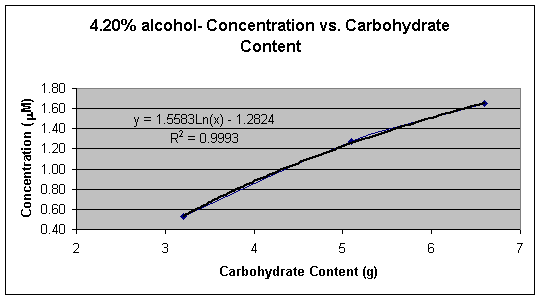
Graph 4: 5.00% alcohol � Concentration (mM) vs. Carbohydrate Content (g)
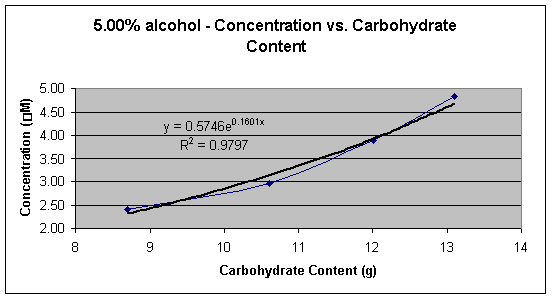
Graph 5: 5.90% alcohol � Concentration (mM) vs. Carbohydrate Content (g)
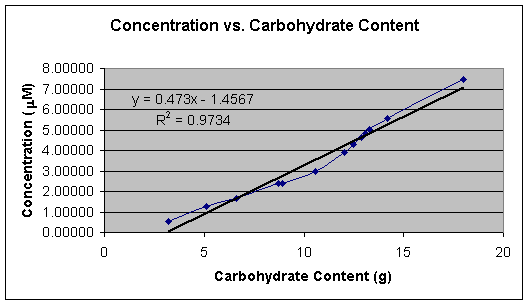
Graph 1 shows the linear calibration curve from 1.00 x 10-6 M to 1.00 x 10-5 M, which was obtained and the equation of the line for the calibration curve was found to be Y = 0.0027x + 0.0006. This equation was then used to calculate the cyanide concentration in each beer sample. Tables 2, 3, 4, and 5 shows the calculated concentrations values for the beer samples. Very pale blue color was obtained for all beer samples, which produced very low absorbance and thus very low concentrations. In Tables 2, 3, 4, and 5 the beers are grouped according to their % alcohol. The lack of effect of % alcohol and the direct effect of grams of carbohydrates on cyanide presence is shown in Graphs 2, 3, 4, and 5. In Graph 6 all the beer samples with varying carbohydrate content are included showing a direct correlation with grams of carbohydrates and amount of cyanide present.
By comparing the experimentally determined cyanide concentration and the grams of carbohydrates present in the beer samples the hypothesis that the cyanide concentration is directly correlated to the carbohydrate concentration is supported. All fourteen beer samples followed the trend that a higher carbohydrate concentration leads to a higher cyanide concentration. This observation is consistent with the fact that the cyanide in the beer is present in a cyanogenic glucoside, a carbohydrate.
Graphs 2, 3, 4, and 5 show the effect the carbohydrate content on cyanide concentration while the % alcohol is held constant. All four different percent alcohol groups produced similar graphs with similar correlation demonstrating that the % alcohol of the beer has a small or no significant effect on the concentration of cyanide present in the sample.
Graphs 2 and 3 demonstrate a logarithmic trend that occurs possibly due to the loss of HCN in the vapor phase during the removal of alcohol from the beer or due to the reduction of malt to reduce the alcohol content. When alcohol is removed from beer HCN is lost with ethanol in the vapor phase during heating or is reduced naturally when that amount of malt in the beer is reduced. Graphs 4 and 5 demonstrate an exponential trend that occurs possibly due to that there is no loss of HCN. In graphs 4 and 5 no alcohol is removed therefore the beer contains HCN, the by-product from alcohol formation, as well as the cyanogenic glucoside, which means that the average ratio of mol of cyanide per mol of glucoside is larger than 1.0 thus creating an exponential trend.
Graph 6 is of cyanide concentration vs. carbohydrate content regardless of % alcohol. This graph�s small deviations from the linear trend demonstrate that % alcohol has only a small effect on the cyanide levels in the beer. Graph 6 also shows that carbohydrate content is the main contributor to cyanide content by demonstrating the strong correlation between the amounts of cyanide and carbohydrates when % alcohol is ignored.
The result of this study showed that the Microdiffusion Spectrophotometric Method is ideal for detecting very low levels of cyanide in beers or other beverages. The cyanide levels found in the beer were substantially lower than the detection levels of many other methods. Also it is shown that this method is able to separate the cyanide from a complex matrix, in which cyanide is incorporated into a complex cyanogenic glucoside. This method has a lower detection limit than most other methods due to the extreme sensitivity of the indicator used and its ability to extract cyanide form complexes to form free cyanide.
The low detection limit of this method is important because due to the toxicity of cyanide the concentration of cyanide in beverages is kept to a minimum. The concentration of cyanide experimentally determined in beer samples ranged from 0.01364 mg/L to 0.1939 mg/L, which corresponds to approximately 0.00484 mg/12 oz. can to 0.06811 mg/12 oz. can. These levels are very low in comparison to the lowest lethal dose of cyanide for humans, which is equal to 2.857 mg/kg of body weight [3]. The lethal dose in humans is approximately equal to 194.39 mg of cyanide for a 150 Ibs. person, which is a great deal larger than the amount that could possibly be ingested by drinking beer.
[1] Kouichiro, Tsuge; Kataoka, Mieko; Seto, Yasuo. Rapid Determination of Cyanide and Azide in Beverages by Microdiffusion Spectrophotmetric Method. Journal of Analytical Toxicology. 2001. Vol. 25 (4), 228-236.
[2] Kruse, J. M.; Mellon, M.G.. Colorimetric Determination of Cyanide and Thiocyanate. Anal. Chem.. 1953. Vol. 25, 446-450.
[3] Potassium Cyanide; MSDS No. 19350; Fisher Scientific: Fairlawn, NJ, February 6, 2006. https://fscimage.fishersci.com/msds/19350.htm (accessed 4/26/06).
[4] Shayo, N B; Nnko, S A M Gidamis, A B Dillion, V M. Assessment of Cyanogenic Glucoside (cyanide) Residues in MBEGE - an Opaque Traditional Tanzanian beer. International Journal of Food Sciences & Nutrition. 1988. Vol. 49, 333-338.
[5] Zheng, Anping; Dzombak, David; Luthy, Richard; Sawyer, Bernard; et al. Evaluation and Testing of Analytical Methods for Cyanide Species in Municipal and Industrial Contaminated Waters. Environ. Sci. Technol. 2003. Vol. 37, 107-115.
©